What is Sodium Laureth Sulfate?
In personal care and cleaning products, few ingredients raise as many questions as what is sodium lauryl sulfate and its closely related compound, what is sodium laureth sulfate. Both Sodium Lauryl Sulfate (SLS) and sodium laureth sulfate are widely used surfactants responsible for creating foam and lifting dirt, oils, and residue from surfaces and skin. However, their safety, differences, and proper use continue to be widely discussed by consumers, manufacturers, and cleaning professionals alike.
These surfactants are cornerstones in a myriad of products, from shampoos and toothpaste to soaps and laundry detergents, lauded for their ability to create the foamy, sudsy appearance many associate with cleanliness. Yet, the discussion surrounding their safety and environmental impact remains nuanced and complex.
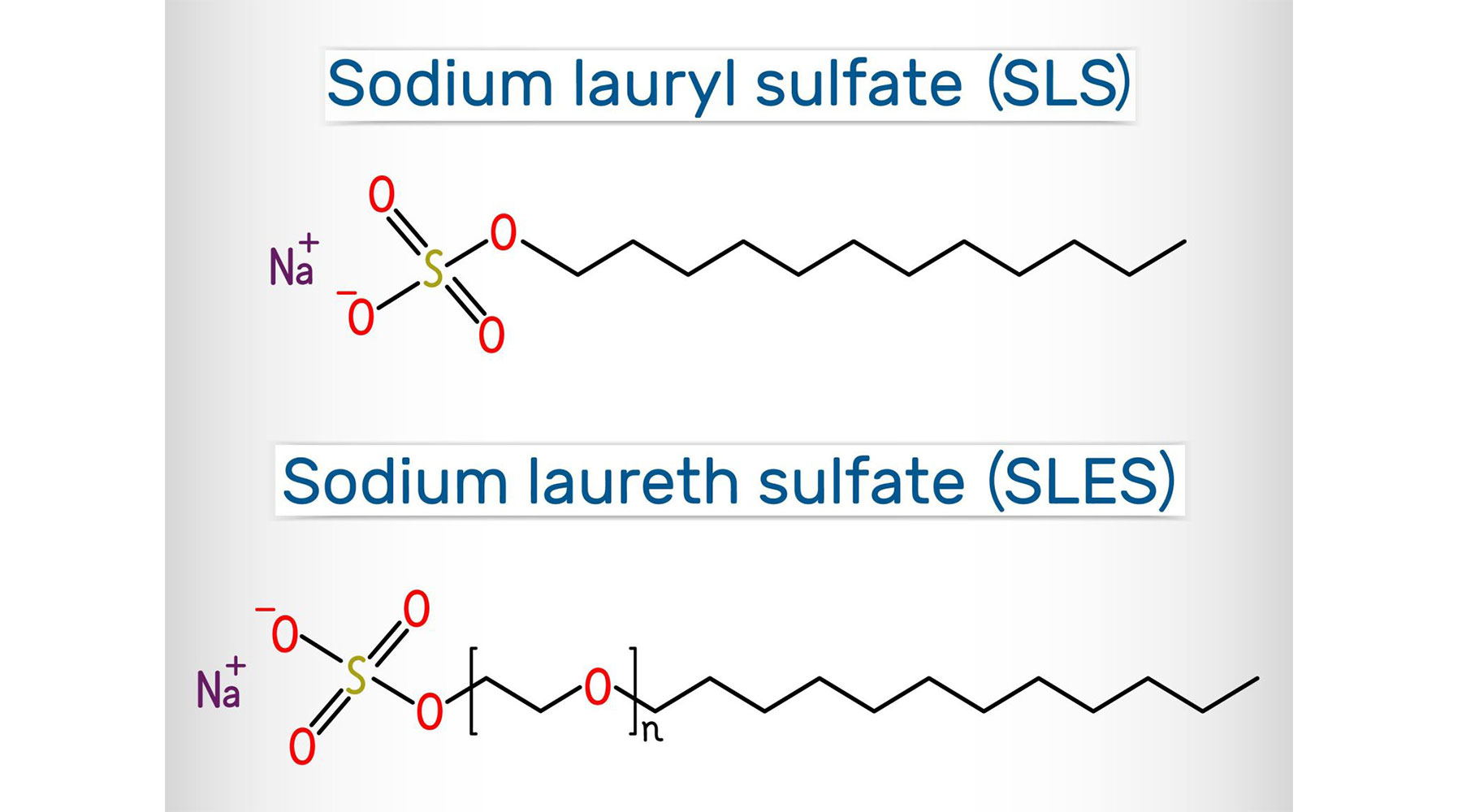
Is There a Difference Between Sodium Lauryl Sulfate and Sodium Laureth Sulfate?
SLS is renowned for its cleaning prowess, enhancing a product’s ability to tackle dirt and oil while delivering the satisfying lather consumers often equate with effectiveness. Despite widespread rumors, SLS is not a carcinogen. Research and authoritative sources, including HealthyChild.org, clarify that there is no evidence linking SLS to cancer. However, its potential for skin and eye irritation, alongside its hazardous effects on aquatic life, prompts a reconsideration of its ubiquitous presence in our daily routines.
Sodium Lauryl Sulfate (SLS): A surfactant derived from natural oils like coconut or synthetically from petroleum, SLS is known for its strong detergency and ability to create a rich lather. It is used in a wide range of products, including toothpaste, shampoos, and cleaners, for its effectiveness in removing oils and residues.
Sodium Laureth Sulfate (SLES): Similar to SLS, SLES is also a surfactant but undergoes a process called ethoxylation, which makes it less harsh on the skin and reduces potential irritation. This modification allows SLES to be used in a broader array of personal care products while remaining effective.
What Is Sodium Laureth Sulfate Made From?
Sodium Laureth Sulfate (SLES) is made from sulfuric acid, lauryl alcohol, and ethylene oxide. Lauryl alcohol is derived from coconut or palm kernel oil, making it a compound with both natural and synthetic elements. The process involves ethoxylation, where ethylene oxide reacts with lauryl alcohol, followed by neutralization with sulfuric acid.
This process increases the molecule’s size, reducing its potential for skin irritation compared to Sodium Lauryl Sulfate (SLS).
7 Uses for Sodium Lauryl Sulfate
Sodium Lauryl Sulfate (SLS) is a powerful surfactant that helps break down oils and grease so they can be rinsed away with water. Because of its strong cleansing and foaming properties, SLS is commonly found in personal care products as well as industrial and commercial cleaning solutions.
1. Shampoos and hair care products
SLS removes oil, dirt, and product buildup from hair while creating a rich lather.
2. Body washes, soaps, and facial cleansers
It lifts oils and impurities from the skin, allowing them to be washed away.
3. Toothpaste and oral care products
Sodium lauryl sulfate helps create foam, improving toothpaste distribution across teeth.
4. Household cleaning products
SLS is used in dish soaps, surface cleaners, and floor cleaners due to its grease-cutting ability.
5. Commercial and industrial cleaning solutions
In professional settings, SLS is used in heavy-duty degreasers for kitchens, restrooms, and high-traffic areas.
6. Laundry detergents
It helps loosen and remove dirt, oils, and stains from fabrics.
7. Car wash and automotive cleaners
The foaming and grease-cutting properties of SLS make it effective for removing road grime.
Health and Environmental Concerns of Sodium Lauryl Sulfate
While SLS and SLES are effective, they have raised health and environmental concerns.
Skin and eye irritation: Both ingredients may cause irritation, especially at higher concentrations or with prolonged exposure.
1,4-Dioxane contamination: SLES manufacturing may introduce trace amounts of 1,4-dioxane, emphasizing the need for strict purification standards.
Aquatic toxicity: SLS can be harmful to aquatic life when released into waterways.
EWG’s stance on SLES: The Environmental Working Group emphasizes usage context and supports products meeting EWG VERIFIED™ standards.
Alternatives and Safer Choices to Sodium Lauryl Sulfate
In response to these concerns, consumers and manufacturers are turning to safer alternatives.
Plant-based surfactants: Ingredients such as decyl glucoside, lauryl glucoside, and coco-glucoside are biodegradable and gentler on skin.
EWG VERIFIED™ products: These meet strict standards for health and transparency.
Label vigilance: Reading ingredient labels helps consumers avoid SLS and SLES if desired.
Navigating the Landscape of Personal Care Ingredients
Understanding SLS and SLES supports better decision-making around health and sustainability.
Knowledge empowers consumers to advocate for safer products and responsible manufacturing.
Conclusion
While SLS and SLES remain effective surfactants, growing awareness has increased demand for gentler alternatives.
At The Cleaning Station, transparency helps customers choose products aligned with health and environmental values.
FAQs
What exactly are SLES and SLS?
SLES and SLS are surfactants that help remove dirt and oil by lowering surface tension.
Are SLES and SLS safe to use?
They are considered safe within regulated concentration limits, though irritation is possible.
Is Sodium Laureth Sulfate harmful or not?
SLES is generally milder than SLS, with modern processes reducing contamination risks.
How can I identify products containing SLES or SLS?
Check ingredient labels for “Sodium Laureth Sulfate” or “Sodium Lauryl Sulfate.”
Are there alternatives to SLES and SLS?
Yes, plant-based surfactants like coco-glucoside offer gentler cleansing options.
Recent Posts
-
High-Touch Surface Cleaning Checklist & Guide for Facilities
Overview: What This Guide Covers This guide provides a complete, ready-to-use high-touch surface cle …Jan 16, 2026 -
Facility HVAC Cleaning Checklist: What to Clean, When, and Why
The Dust You Don't See In many facilities, floors get cleaned, windows shine, and restrooms are sani …Jan 09, 2026 -
Fragrance-Free vs Scented: Build an Allergy-Safe Cleaning Program that Still Works
When "Clean" Becomes a Complaint You know the scene: your custodial team disinfects the office overn …Dec 05, 2025





